ABOUT
Breaking Through The Performance Limit Of Titanium Alloys, The Intrinsic Fracture Toughness Of Titanium
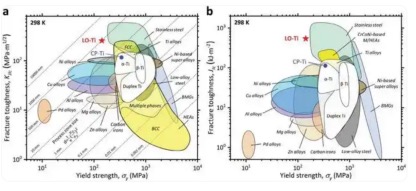
“What’s exciting is that the fracture toughness of low-oxygen titanium exceeds all commercially pure titanium and titanium alloys reported so far, and even exceeds most metal materials.” Professor Han Weizhong of Xi’an Jiaotong University said.
Recently, he and his team successfully broke through the limit performance of titanium and titanium alloys, reducing the oxygen impurity content of commercially pure titanium from 0.14wt.% to 0.02wt.%, and increasing its fracture toughness from 117MPa∙m^1/2 to 255MPa∙m^1/2.
Through this study, they also revealed for the first time the ultra-high intrinsic fracture toughness of titanium, breaking the traditional cognition that the fracture toughness of titanium and titanium alloys is less than 130MPa∙m^1/2, proving that low-oxygen titanium is one of the most resilient metal materials known to date.
In general, this achievement has brought important inspiration for the design of high-strength and high-toughness titanium alloys.
At present, in the field of aerospace, in order to promote the application of titanium alloys under certain safety-critical load conditions, people have adopted the design idea of controlling oxygen content in titanium alloys, which can not only improve the fracture toughness of titanium alloys, but also commercialize related products.
For example, damage-tolerant Ti-6Al-4V (TC4 DT) alloy and ultra-low interstitial (ELI) Ti-6Al-4V alloy have been widely used.
However, for the current damage-tolerant titanium alloy, the oxygen content in it is still at a high level, resulting in its fracture toughness being limited to below 130MPa∙m1/2.
To further improve the application range of classic titanium alloys such as Ti-6Al-4V, it is necessary to improve its service safety. Subsequently, by further reducing the content of oxygen impurities, the fracture toughness of the titanium alloy will be able to achieve a leapfrog improvement.
In fact, almost all close-packed hexagonal structure metals, including titanium, zirconium, magnesium, zinc, etc., which are currently widely used, have the phenomenon that <c+a> dislocations are difficult to activate or have poor mobility.
This makes their plasticity and fracture toughness far lower than most face-centered cubic structure metals, resulting in limited application range.
Therefore, the subsequent alloying design scheme can be used to promote the activation of deformation twins in large quantities.
Then, the dense activation of <c+a> dislocations can be promoted through twin boundaries, thereby significantly improving the mechanical properties of close-packed hexagonal metals.
It Is Expected To Greatly Improve The Deformation Ability Of Close-Packed Hexagonal Metals
Han Weizhong said that he and his team have been paying attention to the influence of dissolved oxygen on the properties of metal materials for many years. A few years ago, they conducted research on the oxygen embrittlement mechanism of vanadium, niobium and tantalum, the fifth sub-group refractory metals.
The study found that the dissolved oxygen in metal materials is easy to combine with the vacancies generated by dislocation movement during deformation to form oxygen-vacancy complexes.
The oxygen-vacancy complex can strongly pin dislocations and promote the formation of deformation micropores, which will cause the oxygen embrittlement phenomenon of the fifth sub-group refractory metals.
In order to achieve a good transformation of the dissolved oxygen embrittlement phenomenon, the research team invented a technology called metal surface gradient oxygen permeation.
That is, by treating metals that are easy to absorb oxygen in a high-temperature oxygen atmosphere, oxygen is allowed to diffuse from the metal surface to the inside of the metal.
During this period, an oxygen concentration gradient from the surface to the inside of the metal will be formed, which will make the surface of the metal material hard, while the core of the metal material can maintain toughness.
In this way, a metal material with both high strength and high toughness can be obtained, and the surface wear resistance of the metal can also be improved.

In fact, metal surface oxygen permeation technology is similar to carburizing technology and nitriding technology, and is a new metal material surface strengthening technology.
When using the metal surface gradient oxygen permeation technology to treat high-purity titanium and perform tensile deformation, they found that the deformation characteristics of the outer gradient oxygen permeation zone and the core low-oxygen zone of the sample showed very significant differences.
Specifically: the core low-oxygen zone will produce a large number of deformation twins, while the gradient oxygen permeation zone near the surface does not form deformation twins due to the relatively high oxygen content.
This shows that the oxygen content has a huge impact on the deformation twinning tendency of titanium, that is, when the oxygen content is low, deformation twins are more likely to occur.
According to the research team, they have been studying the ductile-brittle transition of metal materials in recent years.
As for the sudden decrease in the plastic deformation ability of body-centered cubic metals after the temperature drops, they focused on the microscopic mechanism behind it.
The research results show that the ductile-brittle transition of body-centered cubic metals is closely related to the efficiency of dislocation sources. That is, its determining factor is whether enough movable dislocations can be generated in time inside the metal material to coordinate the deformation during deformation.
At the same time, for the efficiency of dislocation sources, it is determined by the relative mobility of screw dislocations and edge dislocations.
Below the ductile-brittle transition temperature, the mobility of screw dislocations is very poor, while edge dislocations are easy to slide, resulting in the restriction of the mobility of the entire dislocation line.
More importantly, in the case of such uncoordinated movement, the dislocation line cannot be converted into an efficient dislocation source, so it is difficult to achieve effective self-multiplication of dislocations.
At this time, since the number of movable dislocations that can coordinate deformation is too small, once the ambient temperature drops below a certain temperature, the body-centered cubic metal will undergo a sudden ductile-brittle transition.
It can be seen that the toughness and brittleness of metal materials are closely related to the relative mobility of screw dislocations and edge dislocations.
Relying on this discovery, the research team has found a new idea for regulating the deformation ability of close-packed hexagonal metals.
Researchers said that close-packed hexagonal metals such as titanium, zirconium and magnesium have low lattice symmetry.
Usually, the slip of the cylindrical or basal plane <a> is easier to start, while the slip of the conical plane <c+a> is difficult to start.
This will make the close-packed hexagonal metal unable to meet the Taylor-von-Mises criterion, and ultimately lead to the overall weak deformation ability of the close-packed hexagonal metal.
In fact, the reason why the slip of the conical plane <c+a> dislocation is difficult to start is due to its own reasons. Since the <c+a> edge dislocation can be easily decomposed into the cylindrical and basal planes, its mobility is very poor.
The <c+a> screw dislocation is relatively easier to slide, so in close-packed hexagonal metals, people usually observe long straight <c+a> edge dislocations and very short <c+a> screw dislocations.
This shows that the mobility of the two dislocations is very different, and the dislocations that are difficult to slide are usually retained. This is also similar to the phenomenon of long straight screw dislocations often seen in brittle body-centered cubic metals.
For the edge component and screw component of the pyramidal <c+a> dislocation, there is a large difference in their mobility, so their self-proliferation ability is very weak, which will lead to a lack of sufficient pyramidal <c+a> dislocations to coordinate the <c> axis deformation during deformation.
In order to increase the number of <c+a> dislocations, the strategy of increasing the number of <c+a> dislocation sources can be adopted.
It is worth noting that the team happened to find in previous studies that the twin boundaries in zirconium can emit <c+a> dislocations. Based on this, they promoted twin deformation by reducing the oxygen content.
At this time, a large number of twin boundaries can be used as dislocation sources, which can stimulate high-density <a> and <c+a> slip, and thus are expected to greatly improve the deformation capacity of hexagonal close-packed metals.
The Fracture Toughness Value Is Stable At 255mpa∙M1/2
In fact, at the beginning of the study, they did not focus on the effect of oxygen content in titanium on fracture toughness, but focused on the relationship between the microstructure and fracture toughness of pure zirconium with a close-packed hexagonal structure.
After discovering the important effect of oxygen on the twinning of hexagonal close-packed metals, the research team began to try to use low-oxygen titanium as a model material to study its deformation mechanism and fracture toughness.
During the test, they encountered a situation they had never encountered before: compared with commercial pure titanium, the crack propagation rate of low-oxygen titanium samples during the loading process was very slow.
So they re-examined the research plan. Later, they decided to start from the perspective of revealing the intrinsic fracture toughness of titanium, and found that low-oxygen titanium is one of the toughest metal materials known.
At the same time, there are two questions that must be answered:
First, how to measure a fracture toughness value that meets the fracture mechanics standard and can be widely recognized by peers?
Second, why does low-oxygen titanium have such high fracture toughness? What is its inherent toughening mechanism?
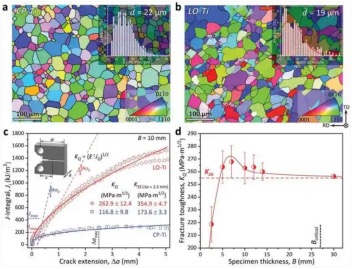
In order to measure the standard fracture toughness value, they began to study the relationship between the fracture toughness of low-oxygen titanium and the thickness of the sample.
In detail: The research team prepared multiple samples with thicknesses ranging from 2.5mm-30mm, and measured the corresponding fracture toughness values respectively, and then studied the trend of the fracture toughness of the samples with thickness.
The results show that the fracture toughness value of low-oxygen titanium can be stabilized at 255MPa∙m1/2. The thickest thickness of the sample they used is 30mm, which exceeds the minimum thickness of 27mm required by the fracture mechanics standard.

For comparison, they conducted standard tests on commercial pure titanium and found that the fracture toughness of commercial pure titanium was only 117MPa∙m1/2, which is far lower than the fracture toughness of low-oxygen titanium.
The biggest difference between the two pure titanium alloys mentioned above is the different content of oxygen impurities. The oxygen content in commercial pure titanium is about 7 times that of low-oxygen titanium, which indicates that the oxygen impurity content is the main reason for the difference in fracture toughness between the two.
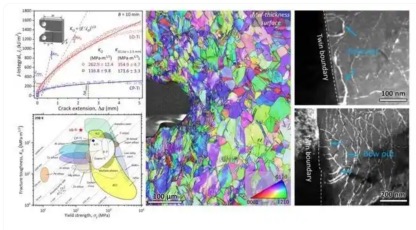
Subsequently, they further analyzed the dislocation structure characteristics in the two titanium alloys. It was found that commercial pure titanium mainly contains <a> dislocations and very few <c+a> dislocations.
In contrast, the crack tip area of low-oxygen titanium activates a high density of <a> dislocations and can start a large number of <c+a> dislocations, and these <c+a> dislocations are emitted from the twin boundaries. This shows that the twin boundaries are the source of <c+a> dislocations.
For this reason, low-oxygen titanium forms a unique progressive toughening mechanism. When the oxygen content is reduced, the crack tip deformation twins of low-oxygen titanium can be activated in large quantities.
Subsequently, the twin boundaries will emit high-density <c+a> dislocations, which can significantly increase the deformation density and plastic zone size at the crack tip, thereby effectively passivating the crack and ultimately allowing low-oxygen titanium to have ultra-high fracture toughness. This officially concludes this study.
A related paper titled “Uncovering the Intrinsic High Fracture Toughness of Titanium via Lowered Oxygen Impurity Content” was published in Advanced Materials[1].
The reviewers of this paper said that compared with high-toughness metal materials such as stainless steel, titanium usually exhibits lower fracture toughness, which reinforces the stereotype that close-packed hexagonal metals have poor inherent toughness.
However, the results of this study on low-oxygen titanium challenged this stereotype. It shows that close-packed hexagonal metals have high fracture toughness potential and can be comparable to stainless steel.
In the future, the research team hopes to apply the low-oxygen strategy to the design and manufacture of high-strength and high-toughness titanium alloys.
For the widely used Ti-6Al-4V alloy, they plan to develop a new sample preparation process, hoping to significantly reduce the oxygen impurity content in the Ti-6Al-4V alloy.
This is expected to help promote the large-scale initiation of deformation twins at the crack tip and further activate the <c+a> dislocation, thereby significantly improving the fracture toughness of the Ti-6Al-4V alloy, expanding its application range and improving service safety.
In addition, the alloy composition of titanium alloys can be designed to promote the initiation of deformation twins or <c+a> dislocations at the crack tip through specific alloying elements, helping to achieve a synergistic improvement in the strength-fracture toughness of titanium alloys.
Keywords:
titanium alloys,low-oxygen titanium,commercially pure titanium,titanium,high-purity titanium,damage-tolerant titanium alloy,ultra-low interstitial (ELI) Ti-6Al-4V alloy,Ti-6Al-4V,Ti-6Al-4V (TC4 DT) alloy
We Exist to Make Your Projects Successful
Fill out the form below and someone from our team will be in touch with you!
FOTMA Delivers Professional Ultra-Dense Solutions, Lighter on Cost, Heavier on Performance.
Hubei Fotma Machinery Co. Ltd.
Wechat / Whatsapp / Mobile:
+86 13995656368, +86 13907199894
Tel: +86-27-67845266
Email: bunny@fotma.com, export@fotma.com
Address: Guanggu Avenue 52#, Hongshan, Wuhan,
Hubei province, P.R.China. 430074